An Organic Light Emitting Diode (OLED), also Light Emitting Polymer (LEP) and Organic Electro Luminescence (OEL), is any Light Emitting Diode (LED) whose emissive electroluminescent layer is composed of a film of organic compounds. The layer usually contains a polymer substance that allows suitable organic compounds to be deposited. They are deposited in rows and columns onto a flat carrier by a simple "printing" process. The resulting matrix of pixels can emit light of different colors.
Such systems can be used in television screens, computer displays, small, portable system screens such as cell phones and PDAs, advertising, information and indication. OLEDs can also be used in light sources for general space illumination, and large-area light-emitting elements. OLEDs typically emit less light per area than inorganic solid-state based LEDs which are usually designed for use as point-light sources.
A significant benefit of OLED displays over traditional liquid crystal displays (LCDs) is that OLEDs do not require a backlight to function. Thus they draw far less power and, when powered from a battery, can operate longer on the same charge. Because there is no need for a backlight, an OLED display can be much thinner than an LCD panel. Degradation of OLED materials has limited their use.
A typical OLED is composed of an emissive layer, a conductive layer, a substrate, and anode and cathode terminals. The layers are made of organic molecules that conduct electricity. The layers have conductivity levels ranging from insulators to conductors, so OLEDs are considered organic semiconductors.
The first, most basic OLEDs consisted of a single organic layer, for example the first light-emitting polymer device synthesised by Burroughs et al. involved a single layer of poly(p-phenylene vinylene). Multilayer OLEDs can have more than two layers to improve device efficiency. As well as conductive properties, layers may be chosen to aid charge injection at electrodes by providing a more gradual electronic profile, or block a charge from reaching the opposite electrode and being wasted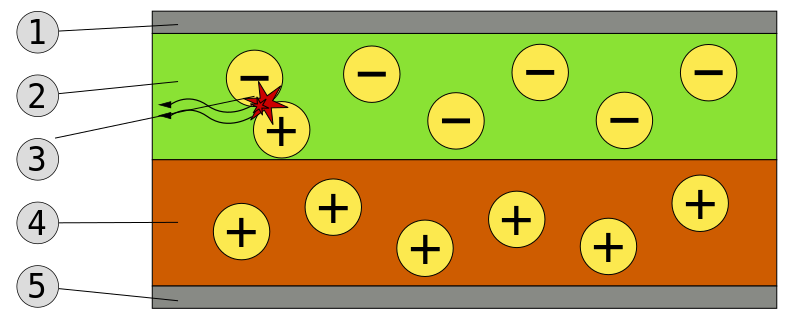
A voltage is applied across the OLED such that the anode is positive with respect to the cathode. This causes a current of electrons to flow through the device from cathode to anode. Thus, the cathode gives electrons to the emissive layer and the anode withdraws electrons from the conductive layer; in other words, the anode gives electron holes to the conductive layer.
Soon, the emissive layer becomes negatively charged, while the conductive layer becomes rich in positively charged holes. Electrostatic forces bring the electrons and the holes towards each other and they recombine. This happens closer to the emissive layer, because in organic semiconductors holes are more mobile than electrons. The recombination causes a drop in the energy levels of electrons, accompanied by an emission of radiation whose frequency is in the visible region. That is why this layer is called emissive.
The device does not work when the anode is put at a negative potential with respect to the cathode. In this condition, holes move to the anode and electrons to the cathode, so they are moving away from each other and do not recombine.
Indium tin oxide is commonly used as the anode material. It is transparent to visible light and has a high work function which promotes injection of holes into the polymer layer. Metals such as aluminium and calcium are often used for the cathode as they have low work functions which promote injection of electrons into the polymer layer.
Just like passive-matrix LCD versus active-matrix LCD, OLEDs can be categorized into passive-matrix and active-matrix displays. Active-matrix OLEDs (AMOLED) require a thin film transistor backplane to switch the individual pixel on or off, and can make higher resolution and larger size displays possible. Soon OLED will be market massly, so just hope for it..
OLED The Future TV
Posted by
Technonews
on
3/08/2009 07:57:00 AM